Monday, April 22, 2013
Cheap spectroscopy
I bought a $10 Carolina spectroscope from Merlan. I pointed it at a CFL bulb and took a photo of the spectrum:
Now compare it to the mercury spectrum:
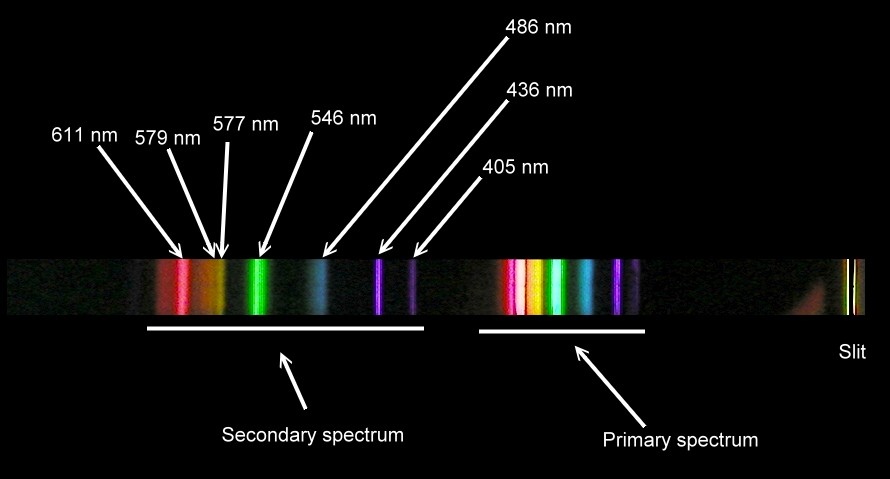
The spectrum peaks are at 435.835 nm (blue), 546.074 nm (green), a pair at 576.959 nm and 579.065 nm (yellow-orange). There are two lines at 404.656 nm and 407.781 nm and a faint line at 491.604 nm.
Compare this to the labelled spectrum of a fluorescent light from this site:
You can actually see the purple line with the spectroscope (it doesn't show up on the photo). When I read the wavelengths from the spectroscope, it matches the numbers shown in the reference photo. This is pretty good for $10!
When I pointed the spectroscope at a halogen lamp, the spectrum was more like a rainbow (full spectrum lighting). This simulates natural sunlight much better than a CFL. This link explains this phenomena very well.
A complete library of atomic spectra is found here. This is very old, but it shows how to take better pictures of spectrometer spectra (click here).
I've also used this to look at the spectrum of a sodium vapour street light.
Subscribe to:
Post Comments (Atom)



No comments:
Post a Comment